AC is converted to dc via a unidirectional device called a diode. A diode can be considered as a one way valve. When certain conditions exist either side of the diode the diode will have a low resistance and will allow voltage through.
At all other times the diode exhibits a high resistance and does not allow anything through.
This means that an ac voltage will permit the diode to conduct each half cycle. This results in a pulse of direct current every half cycle.
This is what happens to an ac voltage on a pipeline at a coating defect. The ac voltage is rectified and the resulting direct current causes corrosion as it leaves the structure and enters the soil.
The “diodes” in this case are created on the steel surface by the chemical reactions that are taking place. The overall process is known as Faradaic Rectification.
Faradaic rectification may be defined as “A component of the current that is due to the rectifying properties of an electrode reaction and that appears if an indicator or working electrode is subjected to any periodically varying applied potential while the mean value of the applied potential is controlled.” In other words it is what you can get at a coating defect that is subject to ac interference whilst a cathodic protection potential is also present.
The big question, however, is how does the ac voltage get onto the pipeline anyway? To understand this we need to consider some basic electromagnetic principles.
- Every electric current has a magnetic field associated with it.
- The magnetic field strength is directly proportional to the magnitude of the electric current.
- In the case of ac current this magnetic field will vary in exactly the same way as the ac current (i.e. 50 times per second in Europe and 60 times per second in the USA)
- A metal conductor placed in a magnetic field will have a voltage induced in it that is directly proportional to the strength of the magnetic field and the speed at which the magnetic field changes.
When we consider voltage induction in buried pipelines and add the knowledge of the corrosion process we can conclude that if a voltage is induced in the pipeline and the pipeline has a resistance (which it always has) then there will be a current flow. If this voltage is converted to direct current then there will be corrosion where the current leaves the pipe. This will occur at the point of lowest resistance i.e. a coating defect. The smaller the defect the greater the corrosion current density. The greater the corrosion current density the greater the metal loss.
Putting all these facts together we can see that high voltage and high current ac cables close to a pipeline with small coating defects has the possibility to cause rapid corrosion at the coating defects.
WHAT CAUSES CORROSION ANYWAY?
There are a wide range of corrosion mechanisms that result in loss of metal from buried pipelines. One thing that they all have in common is that the consequence of the corrosion process is direct current leaves the pipe and that there is a relationship between the magnitude of the current and the metal loss. 1 amp dc for one year will result in 9.1 Kgs of carbon steel consumption.
HOW DO WE KNOW IF A PIPELINE IS AT RISK FROM AC CORROSION?
The scientific community have yet to agree on a single mechanism for ac corrosion. That is probably because they are striving to find one theory whereas there may be several different mechanisms.
External corrosion on buried pipelines is caused by a current exchange between the soil and the pipe. No current exchange, then no corrosion.
Because there is no universal acceptance of the mechanism there is also no consensus on the criteria for ac corrosion.
It would be nice if we could just measure the induced ac voltage and use that to determine whether or not there is a corrosion risk. Unfortunately the voltage by itself is not usually a good indicator of the risk. Knowledge of the soil resistivity close to the coating defect, the pH, the defect size, ac current density and dc current density are also required. These cannot all be measured in the field.
If the ac powerline is more than 110kV and the pipeline is within 150m of the pipeline, and the pipeline is more than 2Km long then the risks of harmful levels of induced ac voltage increase. Areas of particular concern are where there is a change of direction of either the cable or the pipeline route.
As a guideline the following criteria have been used:
- If the ratio between ac and dc current densities is greater than 0.5 then the risk is low
- If the ratio between ac and dc current densities is less than 0.5 the risk is greater
- The risk increases if the ac current density is greater than 30A/m²
- The risk increases if the pipe-to-soil off potential is more positive than -0.850
- The risk increases if the pipe-to-soil off potential is more negative than -0.950
The measurements cannot be made directly on the pipe and they are, therefore, made on a corrosion coupon.
Practical field measurements have shown that by increasing the cathodic protection current density the pH at the surface of the defect changes, and actually accelerates the ac corrosion. So just increasing the cathodic protection current is not always beneficial.
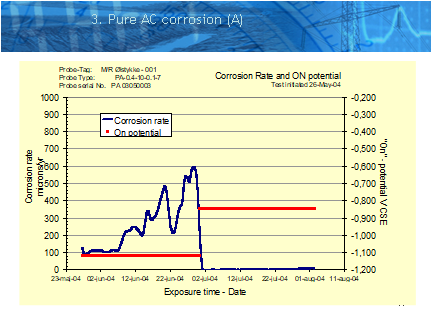
The recommendation of the authors is that a special ER corrosion probe is used to quantify the actual corrosion rates. These probes are placed in areas of risk, and control areas where no risk is perceived, and provide an absolute measure of the corrosion rate regardless of current densities, pH levels etc.
The corrosion probes work by monitoring the extent of corrosion on a known surface area of metal exposed to the soil and connected to the pipe. Using the relationship between resistance and metal surface area they can accurately calculate the metal loss based on the measured resistance. The better quality systems will also measure the on and off potentials and ac and dc current densities to provide additional information. The probes can also be used to validate the effectiveness of any remedial measures.
Another method is to use a mathematical model to predict the ac induction and hence the risks. Not surprisingly this method is popular with the companies that offer the service! Many operators find it comforting to be able to produce calculations and maps to show the risks. It is always worthwhile to consider the true costs and benefits of these models versus the practical application of remedial measures.
CP < 0.85 VDC CP > 0.95 VDC
HIGH DC INTERFERENCE HIGH RISK LOW RISK
HIGH AC INTERFERENCE LOW RISK HIGH RISK